Recently, researchers from the Institute of Physics, Chinese Academy of Sciences, and the Tsientang Institute for Advanced Study, Zhejiang, have unveiled a groundbreaking study revealing anomalous rotational-symmetry breaking in proton arrangement within cyclic hydrogen bonds. Utilizing bond-resolved atomic force microscopy and spectroscopy (BR-AFM/AFS), the team investigated proton behaviors in surface-confined benzimidazole (BI) assemblies. The study highlights the coexistence of localized and quantum-delocalized protons, demonstrating that quantum effects significantly influence proton ordering in hydrogen bonds.
This research reveals proton order-breaking phenomena in cyclic hexamers, where protons exhibit both localized and quantum-delocalized behavior. The study employed Path Integral Molecular Dynamics (PIMD) simulations, showing that quantum tunneling plays a crucial role in proton delocalization. This finding offers new insights into the quantum effects in hydrogen bonding and provides experimental support for designing quantum states in molecular structures.
Moreover, the team demonstrated that a single BI hexamer can reversibly switch between four distinct proton-ordering states. By manipulating adsorption configurations and inducing collective proton transfer, they successfully reversed the chirality of cyclic hydrogen bonds, offering a novel pathway for the development of proton-based quantum materials. This research paves the way for the design of new quantum materials, such as organic ferroelectrics, and offers a fresh perspective on molecular-level proton transfer mechanisms.
This research was led by Professor Yi-Qi Zhang from the Institute of Physics, Chinese Academy of Sciences, and Professor Kehui Wu from the Tsientang Institute for Advanced Study, Zhejiang. The related results were published on December 2, 2025, in Nature Communications (https://doi.org/10.1038/s41467-025-66848-9). Professor Wu is a recipient of the National Outstanding Youth Fund and a leading talent in scientific and technological innovation under the National "Ten Thousand Talents Program." He has focused extensively on surface and interface science of low-dimensional quantum materials, significantly advancing the team's continuous progress and development in the field of quantum materials.
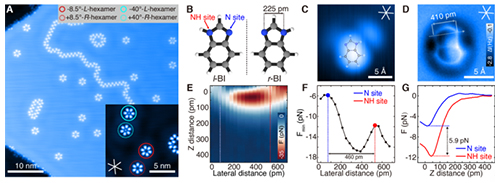
Fig.1 Force spectroscopy characterization of NH and N sites on a single BI molecule.
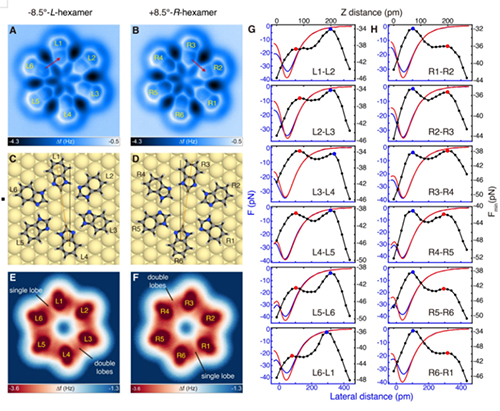
Fig.2 Elucidating H-bonding directionality and proton ordering in a hexamer.
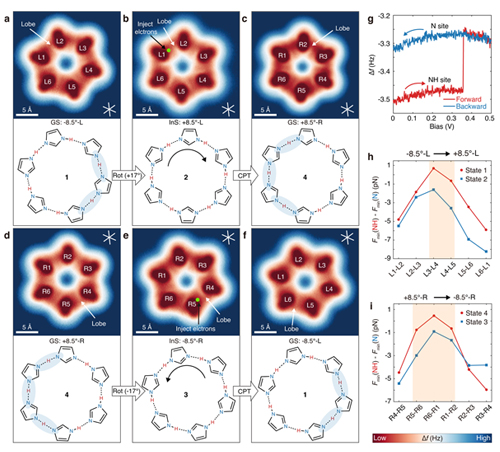
Fig.3 Tip-induced transitions among four proton-ordering states in a single BI hexamer.




